Dr. Noelle LoConte (MD, University of Wisconsin) presents the predictive performance of GemciTest®

Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal cancer, and chemotherapy is a key treatment for advanced PDAC. For several years, Gemcitabine chemotherapy has been an important component of treatment; however, there is no routine biomarker to predict its efficacy. Whereas predictive tests could help clinicians to decide on the best first-line chemotherapy.
GemciTest®, a PCR test based on a nine-gene blood RNA signature, was evaluated in a confirmatory study to measure its sensitivity in predicting patient response to a gemcitabine-based regimen as first-line therapy.
The clinical validation of this in-vitro diagnostic was carried out on 336 patients (mean 68.7- year-old; 37-88) for whom blood was collected from two prospective cohorts and two tumor biobanks.
Patients with a clinical benefit response (OS >8.7 months; PFS ≥3.5 months) identified by GemciTest® (29.7%) had a significantly longer PFS (5.3 vs. 2.8 months) and a longer OS (10.4 vs. 4.8 months). Patients with a positive GemciTest® had a significantly longer PFS (HR=0.53 (95% CI: 0.31–0.92); p=0.023) and OS (HR=0.49 (95% CI: 0.29–0.85); p=0.0091).
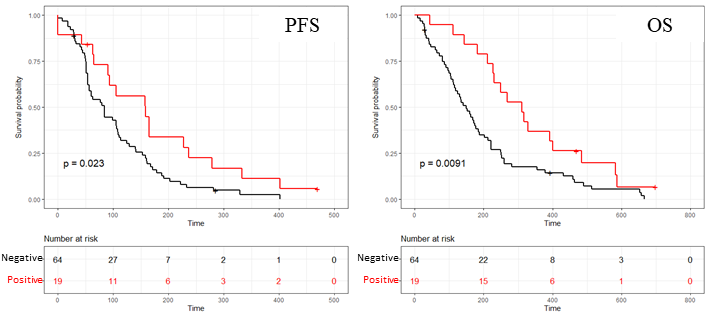
This confirmatory study confirmed the value of using GemciTest® and reveals the potential of a blood-based RNA signature contributing to a personalized therapy in the context of PDAC and allowing for improved survival.
Thus, GemciTest® could improve within 48 hours the benefit/risk ratio of a gemcitabine-based regimen as first-line therapy.
To discover the results of the confirmatory study presented by Dr Noelle LoConte (MD, Associate Professor of Medicine, University of Wisconsin Carbone Cancer Center), please click on the following link:

