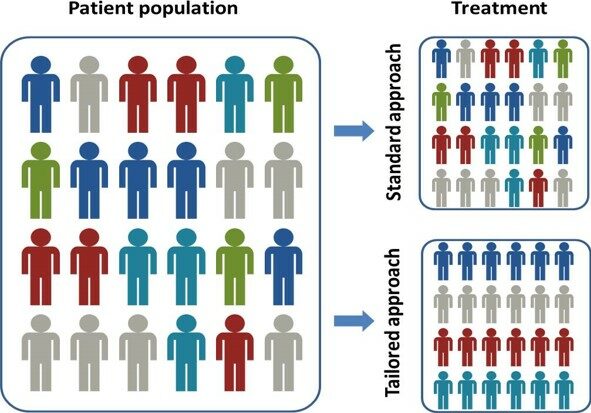
Diagnostics to give the right treatment to the right patient
ACOBIOM develops Diagnostics from R&D to commercialization in growing markets. These innovative blood diagnostics aim to improve the medical care of patients by identifying those who are most likely to benefit from certain treatments. These predictive diagnostics are essential tools for the deployment of precision medicine.
Diagnostics dedicated to precision medicine
ACOBIOM develops innovative diagnostics to predict the individual response of each patient to certain treatments. These blood-based diagnostic tests address diseases with unmet medical needs.
The company’s diagnostics are non-invasive and can be performed from a simple blood sample (liquid biopsy). They are based on the measurement and analysis (via proprietary algorithms) of a patented combination of several biological markers (or biomarkers), of RNA-type.
These blood-based diagnostics have been developed for routine use on platforms already operational in central laboratories and hospitals.
The advantages of ACOBIOM’s diagnostics are to help choose the most appropriate therapy for each patient, to improve the quality of life of patients during treatment, to avoid the appearance of treatment resistance phenomena, to reduce side effects and toxicities, to avoid ineffective, uncomfortable or even harmful treatments and, in general, to improve the medical care of patients.
These diagnostics are essential tools for the deployment of precision medicine and for the personalization of treatments.
Diagnostics to improve medical care for patients in oncology
ACOBIOM proposes its first diagnostics for precision medicine in oncology.
The first example is GemciTest®, an innovative, non-invasive in-vitro diagnostic (IVD) that predicts response to a first-line gemcitabine-based treatment in patients suffering from a form of pancreatic cancer: metastatic pancreatic adenocarcinoma (mPDAC).
Patients identified by GemciTest® have a median survival of 13 months.
The confirmatory study of this predictive diagnosis and its clinical relevance for the implementation of personalized medicine in pancreatic cancer was the subject of a presentation by Dr. Noelle LoConte.
This IVD is commercialized by EUROBIO SCIENTIFIC (Euronext Growth Paris) with which ACOBIOM signed an exclusive distribution agreement.

In parallel, the company is developing other predictive diagnostics in oncology.
In particular, ACOBIOM obtained funding from Bpifrance associated with the R&D program called Oncosnipe®, in which the company will develop diagnostics to predict patient resistance to first-line treatments in pancreatic, breast and lung cancers.
The diagnostics under development will expand ACOBIOM’s diagnostic pipeline and complement the GemciTest® from a marketing and medical perspective.
ACOBIOM, an innovative company recognized for its scientific expertise and the commercial potential of its innovations
In June 2022, ACOBIOM obtained the hi-France label from the AFPC, the French association of competitiveness clusters.
According to the AFPC, “this national labeling process enhances the scientific basis and commercial potential of innovations developed in France by relying on the combined expertise of technological and market experts from the public and private sectors”. The hi-France label is “a symbol of the recognition of the excellence of French innovative companies in which to invest. It guarantees investors the best investment files and facilitates the search for private financing“.
This hi-france label follows the award of a “Seal of Excellence” by the European Commission in 2019, a certificate that recognizes the value of the innovation developed by the company: “this proposal is recommended for funding“.

In addition to its scientific expertise and the value of its innovations, ACOBIOM is positioned on growing markets:
> Companion diagnostics in oncology: from US$ 2.48B in 2020 to US$ 6.60B in 2028 (CAGR: 13%)
> Diagnostics for precision medicine: from US$79.77B in 2022 to US$168.40B in 2028 (CAGR: 13.3%),
> Pancreatic cancer: continuous increase in the number of cases: +2.5 to +3.0% per year.
Sources: Reports and Data, The Insight Partners, WHO-CIRC-Globocan.
This positioning and these innovations will enable the company to ensure its growth in the coming years.
For more information on ACOBIOM, its developments and financial needs, please contact the company’s investor relations department at the following address: investor[@]acobiom.com.

