Essential elements of medical practice and precision medicine
Biomarkers & Diagnostics are the key-elements to diagnose a disease or a pathogenic process, to monitor patients during their treatment, to measure patient response to an exposure or a therapy.
In precision medicine domain, Biomarkers & Diagnostics are the support to predict and monitor patient response to therapies, in other words to identify different sub-populations of patients most likely to benefit from predetermined treatments.
What is a Biomarker?
According to FDA & NIH, a Biomarker (or Biological Marker) is “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions”.
A biomarker can be any biological indicator that can be measured. For instance, biomarkers can be cellular or molecular (DNA, RNA, protein, metabolites). They are measured from a tissue biopsy or a liquid biopsy (blood, urine, saliva…). Other biomarkers (physiological, morphological, etc.) can be also used or measured through clinical or medical imaging.
Biomarkers can be either quantitative or qualitative.
Qualitative biomarkers could be involved in a pathogenic process detection within an yes/no analysis, while quantitative biomarkers are involved in pathogenic process detection with a threshold effect.
Biomarkers are used in research and clinical practice for:
> Diagnosing diseases or predicting risks of disease,
> Monitoring healthy people to detect early signs of disease,
> Determining whether a treatment is efficient or not,
> Targeting specific groups of people for whom a particular drug may be useful,
> Producing safer drugs by predicting the potential for adverse effects earlier,
> Providing researchers with the opportunity of having a global view of the events and changes that are always occurring within a cell.
Biomarkers to develop Diagnostics
Most of diagnostics are based on biomarkers. Every In-Vitro Diagnostics are based on biomarkers.
The first step of a diagnostic development is the identification of one or several biomarkers associated with a normal biological process or a pathogenic one, or with the patient response to a predetermined treatment.
The clinical validation of biological, physiological, or morphological markers will determine which marker or which combination of markers is reliable, relevant and specific to the measurement of predetermined process or the patient response to a therapy.
Molecular biology based on genomic, transcriptomic, proteomic or metabolomic biomarkers helps to develop precise diagnostics, notably in precision medicine.
This precision medicine domain requires the identification and the clinical validation of a huge number of biomarkers to predict disease course, to monitor disease evolution, to identify different sub-populations of patients, and to predict and monitor patient response to most of therapies.
Actually, few therapies based on specific drugs are prescribed thanks to the result of diagnosis. Today, few companion diagnostics are already on the market, exception for breast cancer, colon cancer or melanoma.
Several other medical domains needs the development and the commercialization of new diagnostics. Hence, several therapies, as pancreatic cancer or Alzheimer’s disease, need still the identification of specific biomarkers to establish a pathological biological process.
Moreover, diagnosis of several pathologies (solid tumors, for instance) would be more accepted or performed if they could be performed on the basis of biomarkers from liquid biopsies (blood, urine, saliva…)
Biomarkers in routine: an objective still difficult to reach
Most physicians are used to the role of diagnostic tests to clarify and support their clinical decision making. Increasingly over recent years, the diagnostic process has become more strongly driven by the need to pre-select patients based on drug names or classifications.
This move has come about through a number of factors, which include
> advancing technology (enabling us to measure more specific markers of efficacy),
> a heightened understanding of the disease process,
> a greater appreciation of the uniqueness of an individual’s phenotype at the molecular level.
This move is driven by societal factors as well, most prominent among which is the need to restrict targeted therapies to those patients most likely to benefit. With the advent of precision medicine, the so-called “one-size-fits-all” medical approach is being consigned to history.
The basic promise of personalized medicine is that disease diagnosis and treatment will become ever faster, cheaper, more accurate, and more effective.
The emerging interests of payers in ensuring drugs are administered to the correct patients will likely also shape the association of diagnostics and drug regimen landscape going forward.
Indeed, going from biomarkers to diagnostic used in clinical practice through regulatory affairs is a key challenge. Application of genomic approaches to many malignancies has produced thousands of candidate biomarkers for detection and prognostication, yet very few have become established in clinical practice due mainly to lack of clinical validated outcomes…
The challenge of cancer biomarkers in clinical practice
In cancer treatment, there is a shift from the usual clinical practices to novel approaches. Traditionally, cancer patients were treated with drugs of low toxicity or of high tolerance regardless of their efficacy in a given patient if the benefits of that drug are proven in both experimental and clinical conditions. However, recent advances in basic and clinical research provided opportunities to develop ‘personalized’ treatment strategies.
These novel approaches are intended to identify individualized patient benefits of therapies, minimize the risk of toxicity and reduce the cost of treatment.
The development of dedicated diagnostics to guide the use of targeted therapies in the oncology field offers the opportunity of improved treatment outcomes and reduced exposure to toxicity for many patients. The recent emergence of biomarker strategies for treatment selection and monitoring demonstrates the promise of cancer biomarkers.
There are currently 32 valid biomarkers listed by the FDA across a spectrum of therapeutic areas, with cancer the most prominent.
For biomarkers in cancer application, the challenges as well as the benefits seem tremendous. To make the necessary advances in cancer treatment, a patient’s disease must be monitored in an efficient and effective manner.
Today, treatments may be prescribed to large numbers of patients, despite uncertainty in the degree of their response to therapy. An example of this is the systemic chemotherapy offered to locally patients with advanced pancreatic cancer with response rates ranging from single digit to approximately 30%. The routine use of adjuvant chemotherapy for rectal cancer following neoadjuvant chemoradiation is being questioned. This overtreatment in some cases, or “trial and error” approach in others, is lacking in precision, carries with it side effects, the possible progression of disease, and is seen by many as unreasonably expensive. Ideally, therapeutic biomarkers should be used to guide such critical treatment decisions.

Thousands of papers in the course of biomarker discovery projects have been written, but only few clinically useful biomarkers have been successful validated for routine clinical practice.
The following are the major pitfalls in the translation from biomarker discovery to clinical utility:
1. Lack of making different selections before initiating the discovery phase.
2. Lack in biomarker characterization/validation strategies.
3. Robustness of analysis techniques used in clinical trials.
The selection of useful biomarkers must be carefully assessed and depends on different important parameters, such as on validation trials design, on sensitivity, specificity, predictive value, clinical correlation, etc. Unfortunately, biomarkers with ideal specificity and sensitivity are difficult to identify and to validate in clinics on a number of adequate patients.
Recent technological advances in DNA/RNA, and associated technologies offer the opportunity for the simultaneous analysis of a large number of different biomarkers in a single experiment. In addition to these advances, a non-invasive sampling approach has developed rapidly over the past decade, including a growing interest in liquid biopsies.
A new challenge for biopharma sponsors and diagnostic companies
The successful innovation of targeted therapies and the rise of precision medicine generate a growing demand to obtain accurate and reliable means of identifying in clinical practice patients who will benefit from a predetermined treatment.
Encouraged by regulatory and reimbursement authorities, this trend drawes biopharma companies closer to the world of diagnostic companies than ever before.
Hence, biopharma companies consider options for establishing an accompanying patient-selection diagnostic framework at earlier and earlier stages in development.
Therefore, biomarkers increase significantly their role in defining patient sub-populations as they can measure outcomes in clinical trials as well as in clinical practice.
The passage from biomarker candidate to diagnostic entity is consequently a real challenge for biopharma sponsors and diagnostic companies with a particular bottleneck on the regulatory requirements.
Acobiom’s approach to discover Biomarkers
At technology level, Acobiom’s approach to discover new Biomarkers combines the strength of Next-Generation Sequencing (NGS) investigating gene expression changes and a suite of state-of-art proprietary bioinformatics and biostatistics tools. This suite includes capabilities to proceed omics (big) data on a large scale, machine learning process…
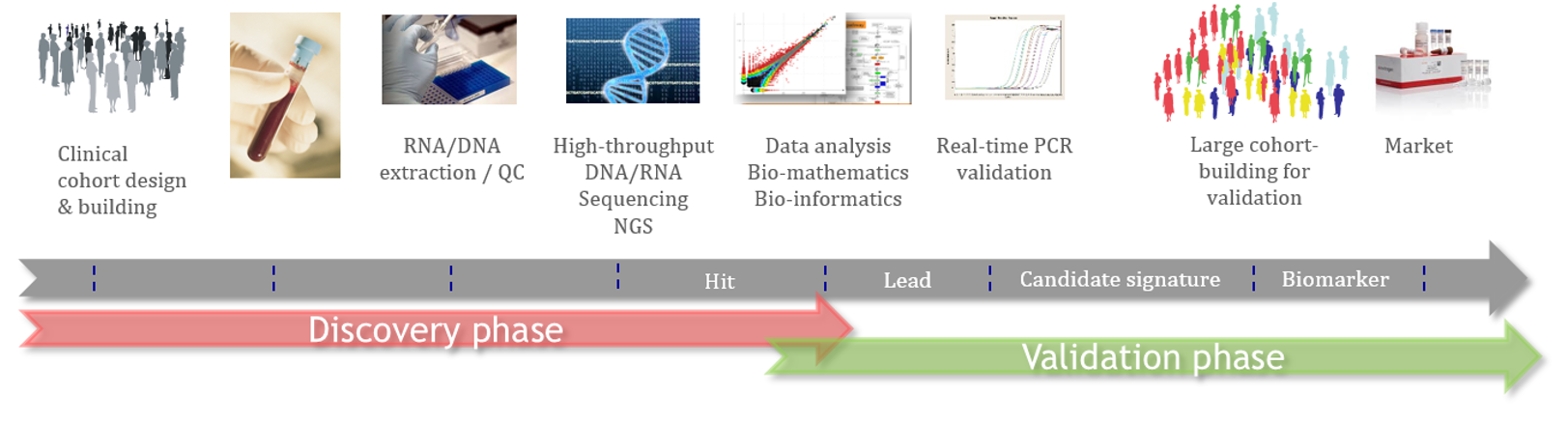
The process to discover new Biomarkers follows a scientific workflow that combines clinical studies, cross-validations by different/complementary technologies, analytical studies and accurate data science tools.
This approach aimes to generate biomarkers/diagnostics that are able to be used by physicians in clinical practice and to be compliant with Health Authorities (EU, FDA) requirements.
Acobiom’s scientific process allows selected biomarkers to go through the bottleneck of selection and validation as well as increasing chances for biopharmas to launch new products on the market.
In this respect, during the course of its R&D and its service activity, Acobiom identified dozens of RNA Biomarkers in correlation with therapies/drugs, cognitive score/impairment, disease detection, evolution and prognosis, immune response, vaccination, infectious diseases, and pharmacogenomics. These Biomarkers were identified in different types of tissues, organs, and for specific biological questions.
In Precision Medicine, Acobiom has been involved in clinical studies (and clinical trials) to identify (Blood) Biomarkers that makes it possible to identify patients most likely to benefit from predetermined treatments.
For Instance, the company developed and validated two qRT-PCT diagnostics:
> GemciTest®, a Diagnostic that can predict patient’s response to Gemcitabine treatment in Metastatic Pancreatic Cancer and prognose a 15-months survival,
> a Diagnostic to prognose a positive 4-year survival in Chronic MyeloMonocytic Leukemia.

