Acobiom announces the CE marking of GemciTest®
GemciTest®, an in-vitro diagnostic that predicts the patient’s response to one of the therapies used to treat pancreatic cancer, benefits now from CE marking, which means it complies with current European regulations in force.
Acobiom develops innovative blood diagnostics in the field of precision medicine. These tests, based on liquid biopsies (blood), aim to give the patient the most appropriate or effective therapy based on certain biological characteristics (genomic markers).

The company, located at the Biopole Euromedicine in Montpellier, applies this approach in the field of cancer, and pancreatic cancer in particular.
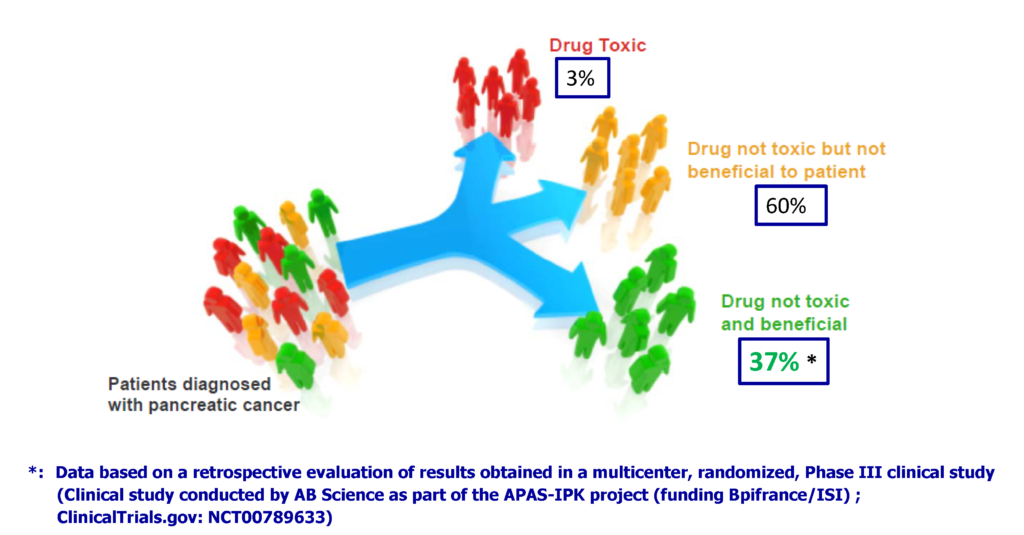
For 80% of patients, pancreatic cancer is diagnosed at an advanced stage and not operable, which generally makes the disease incurable. For these patients, the choice of the best therapeutic solution is therefore crucial and must be made very quickly. The objective is to increase the patient’s survival after the diagnosis of the disease (survival currently estimated at 6 to 7 months).
In a recent clinical study, Acobiom identified a combination of biological markers (biomarkers) in blood that identify patients for whom the use of Gemcitabine (one of the available therapies) in 1st line provides a real benefit. The survival of these patients after diagnosis of the disease was estimated at an average of 15 months.
This combination of blood biomarkers has led to the development of an in-vitro diagnostic, GemciTest®, based on real-time PCR technology.

GemciTest® now benefits from CE marking, which means it complains with current European regulations in force in the field of In-Vitro Diagnostic Medical Devices.
The CE marking of GemciTest® has been filed under the new General Data Protection Regulation (GDPR) and in accordance with the 98/79/EC Directive (more information).
The clients of GemciTest® are centralized laboratories and hospital laboratories, which perform the biological analyses and tests prescribed by physicians and hospital practitioners.
Hence, Acobiom is the 1st company worldwide to commercialize a blood diagnostic that allows therapy to be adapted according to the biological (genomic) profile of patients with non-operable pancreatic cancer.
In addition, the medical and economic interest of GemciTest® in the treatment of patients with pancreatic cancer has attracted the attention of the European Commission by awarding this diagnostic a “Seal Of Excellence” in June 2019.

GemciTest® will thus contribute to improving patients’ quality of life during their treatment by avoiding the prescription of ineffective therapies with significant or harmful side effects.
Since gemcitabine also does not require significant “logistics” and a few days’ stay in a hospital, the use of GemciTest® will also have a positive impact on caregivers and patients’ families.
About Pancreatic Cancer
According to Santé Publique France, 15,000 new cases of pancreatic cancer are expected to be diagnosed in France in 2020. This cancer is one of the few diseases whose incidence and number of deaths are increasing worldwide: +2 to 3% per year, according to WHO. It is the 12th most frequent cancer, but should soon surpass breast cancer to become the 3rd most common cause of cancer death in the European Union and the United States.
About Precision Medicine
The objective of precision medicine is to give the patient the most appropriate or effective therapy according to certain clinical criteria and certain markers (biological or genomic).
The benefits of this targeted therapy approach are:
- Target therapies / Prescribe treatments according to certain patient and disease markers, in contrast to the “trial and error” approach,
- Avoid ineffective treatments or adverse reactions, expensive therapies, sometimes severe toxicity problems, drug resistance phenomena,
- Avoid several treatment lines,
- Improve the quality of life of patients and caregivers,
- Help control health care costs.
About Acobiom
Contact: busdev@acobiom.com

