A new diagnostic to predict the patient response to FOLFIRINOX in pancreatic cancer

Pancreatic cancer is one of the most deadly cancers, with a 5-year survival rate of 12%. For about 80% of cases, the cancer is non-operable, and the objective of the few therapies available is then to extend the patients’ lifespan.
Currently, there are few reliable biomarkers or diagnostics to predict the treatments’ efficacy in pancreatic cancer, to help clinicians quickly choose the most appropriate first-line chemotherapy for each patient.
Among the reference treatments available, FOLFIRINOX is a chemotherapy combining several active molecules (folinic acid, oxaliplatin, irinotecan and 5-fluorouracil). This combination is among the most effective (overall survival: 11.1 months; T. Conroy et al., 2011), but has severe, even harmful side effects.
ACOBIOM has recently developed GemciTest®, a blood PCR test based on a RNA signature (combination of RNA biomarkers), which predicts the patient response to a gemcitabine-based first-line treatment in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC), one of the most common and aggressive forms of pancreatic cancer.
In parallel, on the basis of two independent clinical trials (NCT02818829, NCT03599154), the company carried out a retrospective study on the basis of 164 patients with pancreatic ductal adenocarcinoma (PDAC) at diagnosis and before any treatment (mean age: 68.7 years). These patients received either gemcitabine-based chemotherapy or FOLFIRINOX.
ACOBIOM thus discovered a RNA signature in blood that would identify PDAC patients most likely to benefit from FOLFIRINOX as first-line treatment, leading to an overall survival of more than 2 years for locally advanced PDAC patients.
FolfiTest, the blood-based diagnostic using this new RNA signature, measures the expression level of several genes in blood cells by real-time PCR. Among patients treated with FOLFIRINOX (n=90), those with a positive FolfiTest result (31.1%) had a median overall survival three times greater than those with a negative FolfiTest result.
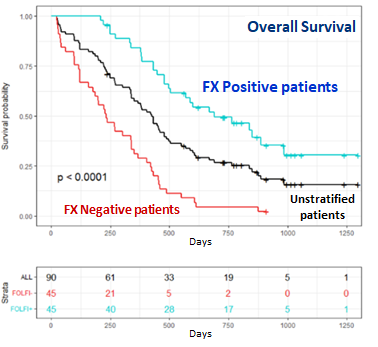
These results show that FolfiTest, a minimally invasive (a simple blood sample is needed) and rapid (less than 48 hours are required to establish the result) diagnostic, would be of major medical interest in predicting the response of PDAC patients to treatment with FOLFIRINOX.
Additional studies are now required to confirm these initial results and enable patients diagnosed with unresectable locally advanced tumors, on the one hand, and those with unresectable metastatic tumors, on the other, to be rapidly and specifically guided towards the best treatment.
Once these confirmatory studies have been completed, FolfiTest can be integrated into the pre-therapeutic assessment already planned in the patient management. Marketed by ACOBIOM distributors, this diagnostic will be promoted to oncologists and used by central or hospital laboratories.
Find out more about FolfiTest in the presentation video

